A Natural History of the Unionidae Mussels
of the Lower Susquehanna River Watershed
Including Commentary on Other Native and Non-native Bivalve Mollusks
SUMMARY
Historical records, particularly those of local malacologist Samuel Steman Haldeman (1812-1880), indicate nine species of native Unionidae bivalves, commonly known as river mussels, have occurred in the waters of the Lower Susquehanna River Watershed in Pennsylvania. One of the nine species, Alasmidonta heterodon, is likely extirpated. Another, Lampsilis radiata, is seriously diminished in range—a fragmented population surviving mostly downriver of Pennsylvania in tidal freshwater areas near the mouth of the Susquehanna in Maryland. Two additional species of unionids, Anodonta implicata and Leptodea ochracea, favor these same tidal waters and occur in the Susquehanna basin only in Maryland.
In the Lower Susquehanna River Watershed, Unionidae communities are comprised of these ten surviving species—Alasmidonta marginata susquehannae, Alasmidonta undulata, Anodonta implicata, Elliptio complanata, Lampsilis cariosa, Lampsilis radiata, Lasmigona subviridis, Leptodea ochracea, Pyganodon cataracta, and Strophitus undulatus. Since 1980, an additional species, Alasmidonta varicosa, has been reported in small numbers, but exclusively outside of Lancaster County. It may have escaped the notice of Haldeman and other nineteenth-century naturalists who conducted the majority of their surveying along the river in Lancaster County and in nearby areas on the east side of the Susquehanna. Anodontoides ferussacianus and Villosa iris have also been reported during the years since 1980, though the status and origins of these species are as yet undetermined. Their arrival as native transplants, particularly as larval parasites on stocked fish, is suspected. The latter species, Villosa iris, appears to be reproducing.
The river mussels are widely regarded to be the most imperiled family of animals in North America. Improved interest in populations of Unionidae bivalves throughout the Lower Susquehanna River Watershed has led to better monitoring of the surviving species and increased concern for their habitat—our waterways.
All Unionidae mussels occurring in Pennsylvania and Maryland are now protected species and may not be collected or harvested.
INTRODUCTION TO THE UNIONIDAE
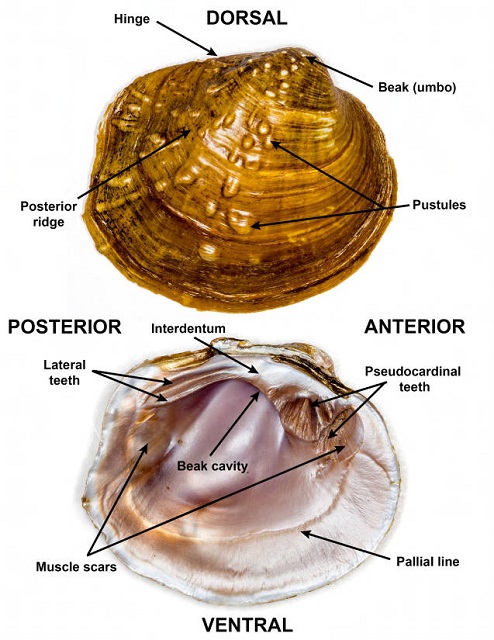
The Unionidae are a family of large freshwater bivalve mollusks often known as river mussels. Adults of all species exceed one inch in length. In some species, older specimens, often decades in age, can be six or more inches in length. Two valves, comprised mostly of calcium carbonate, form the protective outer shell. The inner surface of the shell is covered with a thin surface called the nacre. The color of the iridescent nacre is often a key to species identification. Viewed on the exterior, the valves are joined dorsally near a forward directed beak or umbo. Unionids spend much of their time partially buried in the substratum. The beak of each valve is often worn and white because it is the only portion of the animal exposed to abrasive materials in the current. In some species, a posterior ridge extends from the beak to the posterior-ventral corner of the valves. Internally, the valves often have protruding pseudocardinal hinge teeth anterior to the beak and long thin lateral hinge teeth extending toward the posterior.

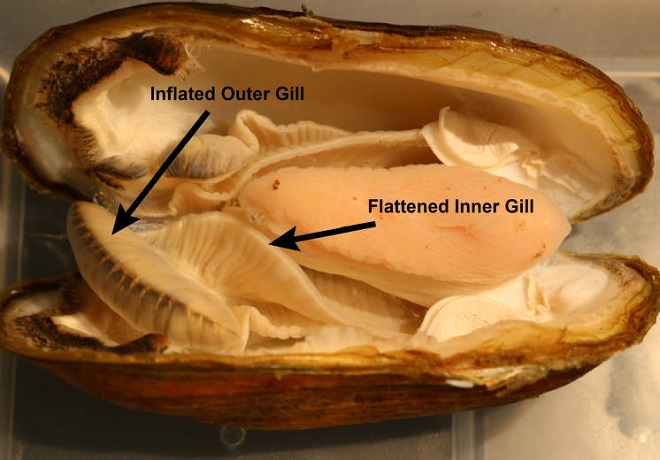
The Unionidae exhibit slow locomotion using a soft “foot” extended ventrally toward the anterior end of the open valves. To the posterior they exchange water through the incurrent and excurrent apertures, also known as the inhalant and exhalant siphons. Motion of the internal gills provides respiration and the transfer of food, plankton, and organic detritus, to the labial palps.
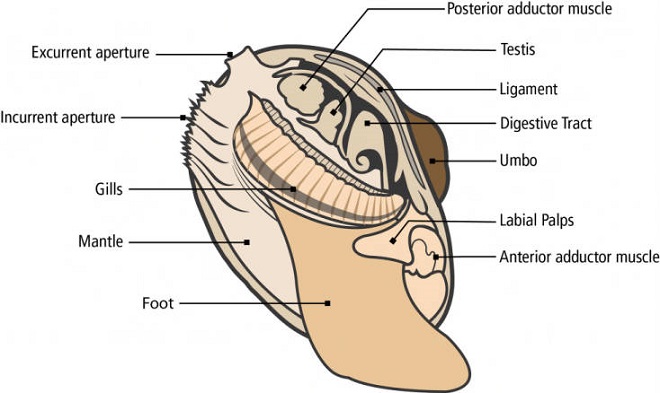

The eggs of the female are fertilized in the gills with sperm pumped in through the incurrent aperture. After hatching, the larvae, known as glochidia, are brooded in the gills. Upon being released by the female, glochidia must quickly become attached to the gills or fins of a preferred species of host fish to survive. Some species of unionids expel their glochidia to the stream bottom. In the case of others, a portion of the female’s body resembling a prey species favored by the host fish is displayed outside the shell like a lure. When a potential host is attracted, glochidia are ejected by the female mussel so that they might be inhaled during respiration by the host fish and trapped in its gills. Successful glochidia encyst on the host for about two to four weeks, causing little if any harm. The parasitic stage of their life cycle complete, they detach and begin life in the substate as juvenile mussels.
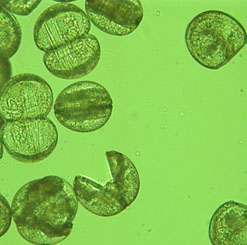


Those glochidia that break free in suitable benthic habitat stand the best chance of survival. Where fish movements are unencumbered, the life cycle of a unionid mussel enhances its ability to remain widely distributed in favorable waters throughout a watershed. Detrimentally, the life cycle can place limits on the success of reproduction in impaired streams, where glochidia can break free and become buried in silt or other unfavorable substrate.
Juvenile unionids that survive the larval stage of their life cycle spend the remainder of their lives in a small geographic area, growing slowly while filtering the nourishment they need from the water column. Collectively, healthy populations of river mussels and other bivalves purify massive volumes of water, effectively reducing turbidity and sequestering nutrients in the streams, rivers, lakes, and ponds they inhabit. In the Lower Susquehanna River Watershed, restoration of communities of native freshwater mussels and clams can help improve water quality for both human use and aquatic life alike. Cleaner water here extends its benefits downstream to Chesapeake Bay, home to important fish nurseries and troubled populations of a well-known and very popular bivalve—the Eastern Oyster (Crassostrea virginica).

THREATS TO THE UNIONIDAE
All native freshwater bivalves—our mussels and clams—are particularly susceptible to habitat and water quality degradation. As a family, the Unionidae are probably the most threatened animals in North America. In the Lower Susquehanna River Watershed, they are seriously diminished in numbers or are absent from streams affected by one or more of the numerous impairment factors that besiege them
MECHANICAL ALTERATION OF STREAM CORRIDORS
Mechanical alterations to stream bottoms and floodplains can negatively impact populations of freshwater mussels. There are several undertakings that are, or were, particularly devastating.
Man-made dams, first those constructed for milling operations, then those built for recreation lakes and hydroelectric energy production, have seriously reduced mussel and clam populations in the Lower Susquehanna River Watershed. Silt and sediment accumulations behind dams often bury suitable substrate for a mile or more upstream of the impounding structure, rendering the bottom of the waterway uninhabitable for bivalves and the many other forms of benthic life that thrive in sand and gravel. After detaching from their host fish, young unionids are particularly susceptible to being buried in silt. Adult mussels find the muddy bottoms of mill ponds and other impoundments inhospitable as well. Because they rely on a host fish to nourish and distribute their parasitic larvae (glochidia), unionids are especially sensitive to the limitations on fish movements created by man-made dams. When dams impede the migrations and other seasonal travels of host fish carrying larval glochidia, contraction and fragmentation of the mussel species’ geographic range can result. In the absence of a suitable substitute fish, loss of a host fish species can lead to the disappearance of any river mussel that relies on it for larval development.

Dredging of sections of the lower Susquehanna for spilled, discarded, and washed-out anthracite coal sediment went on for nearly 100 years and was a particularly active enterprise in the Harrisburg area and in Lake Clarke below Chickies Rock until 1973. The repeated benthic disturbances that accompanied waste coal recovery in these segments of the river may have been the catastrophic factor leading to the demise of Eastern Lampmussel (Lampsilis radiata) and Dwarf Wedgemussel (Alasmidonta heterodon) populations there.
Stream channelization is mechanical modification to stream geomorphology that destroys both fish and river mussel populations by negatively altering substrate composition, water quality parameters, and flow characteristics during periods of both high and low water. In the lower Susquehanna valley, the naturally occurring dynamic of a multi-channeled stream meandering through a floodplain of vegetated wetlands is seldom tolerated. Though misguided, channelization is the favored “remedy”, despite the horrendous damage it causes.

NUTRIENT AND SEDIMENT RUNOFF
As filter feeders, freshwater mussels and clams are renowned for clarifying water by removing organic particulates, but they are intolerant of excessive turbidity. Unionids do not remove silt and sediment in suspension and are especially vulnerable to excessive loads of nitrogen and phosphorus, the nutrients that lead to massive blooms of algal growth. As algae dies and decays, eutrophication and its depletion of dissolved oxygen levels can prove fatal to populations of bivalves, especially in warm weather. For this reason, river mussels have disappeared from Susquehanna valley streams affected by significant volumes of agricultural and urban runoff. The effects are magnified where streamside vegetation has been removed and riparian buffers are lacking.




REDUCED BASE FLOW
In addition to the effects of the silt and nutrients that lead to eutrophication, loss of base flow in streams that drain disturbed landscapes make them more likely suffer severe depletion of dissolved oxygen levels during hot weather. Within a given watershed, progressive reductions in stream base flow can occur as increased volumes of ground and surface waters are removed for human use. This condition is further aggravated when the amount of rainfall infiltrated to recharge groundwater supplies decreases as the volumetric area of the watershed converted to non-porous surfaces such as streets, parking areas, lawns, and roofs increases. In urbanized areas, waterways with reduced flow are susceptible to thermal shock during summertime showers when stormwater drains away from sun-scorched pavement into a stream with an already stressed ecosystem. During periods of drought, a body of water may dry up completely. River mussels can survive for just a short time by retreating into their shell and remaining buried in the substrate, but prolonged exposure to air and sunshine is fatal. While fish may recolonize a segment of desiccated waterway rather quickly, unionids may not readily return.


TOXIC POLLUTANTS
Freshwater mussels may bioaccumulate pesticides and other toxic substances found in their environment. Because unionids, following their larval stage, spend their entire lives in a small geographic area within a single body of water, and because they can live for decades, these invertebrates can be ideal long-term indicators of stream health. For the same reasons, unionids don’t belong on anyone’s menu.

ACID MINE DRAINAGE
Some streams in the Lower Susquehanna River Watershed lack river mussels for self-evident reasons. Those waterways to the north and northeast of Harrisburg that originate in Pennsylvania’s anthracite coal fields run red brown with acid mine drainage—pure poison to aquatic creatures, particularly bivalves whose calcium carbonate shells dissolve in low pH water. A conspicuous example of the effects of this pollution can be found in the upper reaches of Swatara Creek, one of the lower Susquehanna’s largest tributaries. After originating in the southern mining fields of western Schuylkill County, the Swatara supports a limited diversity of aquatic life during its run through the hills of the Ridge and Valley Province. Despite some treatment of acid mine drainage near its source and buffering by inflow from numerous small tributaries in the valley between Blue and Second Mountains, a functional ecosystem with sustainable populations of fish, mussels, and other fauna inhabits the Swatara only downstream of its confluence with Little Swatara Creek, a diluting tributary that drains a portion of the dolomite-rich Great Valley through which the waterway flows along most of the remainder of its course to the Susquehanna River.

INVASIVE SPECIES
An additional threat to freshwater mussels and clams is competition with non-native invasive species. Introduced fishes can displace a favorable species of host for the mussels’ larvae. Recent introductions of invasive Flathead Catfish (Pylodictis olivaris), Blue Catfish (Ictalurus furcatus), and Northern Snakehead (Channa argus) are especially concerning. Zebra mussels (Dreissena species) are a particular concern—possessing the potential to overwhelm and eliminate otherwise healthy populations of unionids. In extreme cases, zebra mussel colonies have encrusted native mussels, crayfish, and other benthic species (Shaw et al. 2004). Early discovery and control of invasive species, particularly zebra mussels, may protect native organisms, including bivalves, and provide financial savings to municipalities, businesses, utilities and other users of freshwater.


HISTORICAL RECORDS
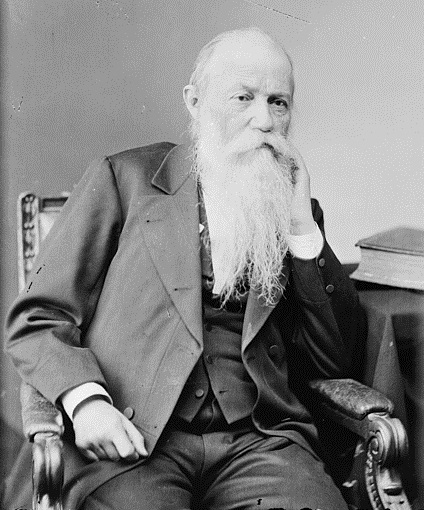
Samuel Steman Haldeman provides an early record of the Unionidae found in the Lower Susquehanna River Watershed in Pennsylvania. Haldeman was born in 1812 at the village of Locust Grove along the Susquehanna, just downstream of Conewago Falls near Bainbridge, Lancaster County. As a boy he studied the wildlife found near his family home, including freshwater mussels and snails. In later years, Haldeman built his own home on the Susquehanna at Chickies Rock. He became a well-known scholar, authoring numerous papers on wide-ranging topics. His best-known works were A Monograph of the Freshwater Univalve Mollusca of the United States, published as a series from 1842 through 1845, and Enumeration of the recent freshwater Mollusca which are common to North America and Europe, 1844. The latter drew the interest of Charles Darwin, who briefly comments on Haldeman’s paper in Origin of Species. Haldeman’s account of the mollusks of the area appears in Rupp’s History of Lancaster County, published in 1844 by I. Daniel Rupp. There, Haldeman describes eight species of river mussels found in the Susquehanna and its “branches” within Lancaster County, Pennsylvania.
-
-
- Unio cariosus—currently known as Lampsilis cariosa
- Unio radiata—currently known as Lampsilis radiata
- Unio complanatus—currently known as Elliptio complanata
- Unio viridis—currently known as Lasmigona subviridis
- Alasmodon undulatus—currently known as Alasmidonta undulata
- Alasmodon marginatus—currently known as Alasmidonta marginata
- Anodon cataractus—currently known as Pyganodon cataracta
- Anodon undulatus—currently known as Strophitus undulatus
-
Later in the nineteenth century, Bruckhart (1869) lists ten species of unionids in the lower Susquehanna watershed in Lancaster County. Active changes in taxonomy during the 1800s may explain the confusing taxa used. Using Clarke and Berg (1959) to edit apparent synonyms from Bruckhart’s list distills his species count to no more than the eight enumerated by Haldeman.
In addition to Haldeman’s eight species, there is an historical record of a ninth— Alasmidonta heterodon, the Dwarf Wedgemussel. Moser (1993) plots the species on the Susquehanna River at Chickies Rock in a location coded as “Historical occurrence, presumed extirpated” at “…Susquehanna River at Columbia, Lancaster County, PA”. The valves of the specimen(s) referred to in Moser’s report are apparently those collected in 1919 by L. H. Streng. They are in the collection of the Philadelphia Academy of Natural Sciences (ANSP 48308). Alasmidonta heterodon is a federally endangered species.
An Eastern Lampmussel (Lampsilis radiata) valve specimen (ANSP 101665) at the Philadelphia Academy of Natural Sciences was collected at York Furnace on September 12, 1910. The ruins of York Furnace are found in southern York County along Otter Creek near its confluence with the Susquehanna River. The small village known as York Furnace lies just downstream of the mouth of Otter Creek opposite the Lancaster County village of Pequea. It is not clear whether the Lampsilis radiata population was in Otter Creek in York County, or in the river near the creek’s mouth, thus within the boundaries of Lancaster County, but a contiguous range in both counties is probable. In the same year the specimen was collected, Holtwood Dam was constructed on the Susquehanna downstream, flooding much of the free-flowing segment of the river at York Furnace as part of the “Lake Aldred” impoundment. Since 1910, silt deposition and waste-coal dredging, along with other habitat and water quality degradation factors, have probably eliminated Lampsilis radiata from most of its suitable habitat in the Pennsylvania section of the lower Susquehanna watershed. A population still exists in tidal freshwater areas at the mouth of the Susquehanna in Maryland. Though believed to be globally secure, Lampsilis radiata is ranked “Critically Imperiled” in Pennsylvania.
Two species of unionids occur in the Susquehanna basin only in areas in or near tidal freshwater at the mouth of the river in Maryland—Anodonta implicata, the Alewife Floater, and Leptodea ochracea, the Tidewater Mucket.
HALDEMAN’S TRANSPLANTS

Throughout the eighteenth and nineteenth centuries, it was common practice for agronomists, botanists, naturalists, and others to collect and transport live plants and animals for introduction into areas outside their native range. Generally, native transplants consisted of species introduced into new areas on their native continent, while non-native transplants were flora and fauna introduced into continents entirely outside of their native range. Both native and non-native transplants possessed the potential to become invasive species. But during these years before the prevalence of any widespread conservation ethos, the threat of significant agricultural or ecological damage from transplanted species went unforeseen. Among those participating in plant and animal introductions, there appears to be little if any awareness of the potential consequences.
NATIVE TRANSPLANTS
The October, 1841, Proceedings of the Academy of Natural Sciences (Volume 1, page 104) record—as part of a presentation on the nomenclature used for the river mussels then known as Unio viridis and Unio tappanianus, and the proposal by Conrad of the species name subviridis for both—that Samuel Stamen Haldeman made known to the members present, “that he had placed some living specimens of Western Unio, Unio rectus, triqueter, circulus, cylindricus, ovatus, and others in the Susquehanna, where no western species has hitherto been found, except U. viridis, Raf.” The mussels Haldeman describes as introduced from the “west”, a term which during the early 1840s often described areas only as far west as the Mississippi and western Great Lakes drainages, were collected from the Ohio River system, probably in Kentucky.
Haldeman, in his presentation to the Academy, makes reference to the similarities shared by “western” specimens of Unio viridis from Kentucky and those from the Atlantic Slope, the latter known by some naturalists as Unio tappanianus. He seems to be convinced that the eastern and “western” specimens are of a single species. Today, Unio viridis and Unio tappanianus are indeed considered a single species—Lasmigona subviridis. Furthermore, Lasmigona subviridisis is recognized not as a “western” species, but as a native of the Atlantic slope, including the Susquehanna watershed. Today, Lasmigona subviridis is absent from the “west”, so how did specimens from Kentucky find their way to the meeting of the Academy of Natural Sciences in 1841. Were they misidentified shells of another similar species? Maybe. But the case of Physella acuta (see the Physella acuta species account by clicking the “Freshwater Snails” tab at the top of this page) demonstrates that the practice of transplanting can complicate the difficulties of differentiating species based on their morphological features and obfuscate their geographic origins. There is the possibility that, similar to the way mussels from the “west” were transplanted into the Susquehanna by Haldeman, live specimens of Lasmigona subviridis had, prior to 1841, been collected from the Atlantic slope, then transplanted into waterways of the “west”, particularly the Ohio River basin in Kentucky.
NON-NATIVE TRANSPLANTS
The February 1846, Proceedings of the Academy of Natural Sciences (Volume 3, pages 14-16) record Professor Haldeman’s reaction to a specimen of Unio crassus presented to him at the meeting, “it is one of those which he placed in the river Susquehanna, in a living state, in the year 1841, a record of which fact will be found at page 104, Vol. 1, of the Proceedings of the Academy. As no western species of Unio except U. viridis, Raf, had hitherto been found in that river, Mr. Haldeman had no doubt that the present specimen was in reality one of those referred to. The growth had been inconsiderable, and the general appearance very little changed. The individuals of this and other species seem not to have survived.” Unio crassus, the Thick-shelled River Mussel, is a declining species native to Europe and is not known to presently inhabit any North American waters.
FRESHWATER BIVALVE MOLLUSKS
Of the Lower Susquehanna River Watershed
UNIONIDAE
The River Mussels
SPECIES STATUS KEY
Federally Endangered-a native species listed by the United States government as imminently in danger of extinction.
PA Endangered-a native species listed by the Commonwealth of Pennsylvania as imminently in danger of extinction or of extirpation as a breeding species in the state.
MD Endangered-a native species listed by the State of Maryland as imminently in danger of extinction or of extirpation as a breeding species in the state.
Federally Threatened-a native species listed by the United States government as under threat to become an endangered species in the foreseeable future.
PA Threatened-a native species listed by the Commonwealth of Pennsylvania as under threat to become an endangered species in the state in the foreseeable future.
MD Threatened-a native species listed by the State of Maryland as under threat to become an endangered species in the state in the foreseeable future.
PA Candidate-an uncommon native species that could, in the future, become listed by the Commonwealth of Pennsylvania as endangered or threatened in the state.
Domain-Eukaryota
Kingdom-Animalia
Phylum-Mollusca
Class-Bivalvia
Order-Unionida
Family-Unionidae
Note: Photographs of river mussel shells on a blue background show two pairs of valves…
-
- A pair of closed valves, showing the exterior of the shell, appears to the left in each photograph. (Two valves are shown in Figure 4 to display female and male.) The left of the closed mussel is the posterior, the right the anterior. The beak and the dorsal surface of the shell are at the top. The ventral edge of the shell is at the bottom.
- A pair of open valves, showing the interior of the shell, appears to the right in each photograph. The left valve is to the left, the right valve to the right. The posterior of the mussel is at the top in this view. Pseudocardinal hinge teeth, if present, are anterior of the hinge along the dorsal edge of the interior of the valves. Lateral hinge teeth, if present, are parallel with the hinge extending toward the posterior of the mussel along the dorsal edge of the interior of the valves.
Alasmidonta marginata susquehannae
Common Name(s): Susquehanna Elktoe
Status:
Rank: Pennsylvania S3 (Vulnerable) and S4 (Apparently Secure), Globally G4 (Apparently Secure) for the species (Alasmidonta marginata)
Host Fish known to occur in the Susquehanna and/or its tributaries: List for interior Alasmidonta marginata, not particularly the Atlantic Slope subspecies A. m. susquehannae—Banded Killifish (Fundulus diaphanus), Brook Stickleback (Culea inconstans), Creek Chub (Semolitus atromaculatus), Creek Chubsucker (Erimyzon oblongus), Northern Hogsucker (Hypentelium nigricans), Shorthead Redhorse (Moxostoma macrolepidotum), Silver Redhorse (Moxostoma anisurum), White Sucker (Catostomus commersoni), Golden Shiner (Notimegonus crysoleucas), Longnose Dace (Rhinichthys cataractae), Mottled Sculpin (Cottus bairdii), Slimy Sculpin (Cottus cognatus), and Rock Bass (Ambloplites rupestris).
Comments:
Haldeman (1844), using the taxa Alasmodon marginatus, described Alasmidonta marginata susquehannae, “… green rayed, cardinal teeth small and thin, posterior extremity of shell truncated. 2 inches.”



Alasmidonta varicosa
Common Name(s): Brook Floater
Status: MD Endangered
Rank: Pennsylvania S1 (Critically Imperiled) and S2 (Imperiled), Maryland S1 (Critically Imperiled), Globally G3 (Vulnerable)
Host Fish known to occur in the Susquehanna and/or its tributaries: Golden Shiner (Notemigonus chrysoleucas), Longnose Dace (Rhinichthys cataracte), Blacknose Dace (Rhinichthys atratulus), Mottled Sculpin (Cottus bairdi), Slimy Sculpin (Cottus cognatus), Redbreast Sunfish (Lepomis auritus), Pumpkinseed (Lepomis gibbosus), Bluegill (Lepomis macrochirus), Margined Madtom (Noturus insignis), Fantail Darter (Etheostoma flabellare), and Yellow Perch (Perca flavescens).
Comments:





Alasmidonta undulata
Common Name(s): Triangle Floater
Status: MD Endangered
Rank: Pennsylvania S3 (Vulnerable), Maryland S1 (Critically Imperiled), Globally G4 (Apparently Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Central Stoneroller (Campostoma anomalum), Common Shiner (Luxilus cornutus), Rosyface Shiner (Notropis rubellus), Fallfish (Semotilus corporalis), Longnose Dace (Rhinichthys cataracte), Northern Hogsucker (Hypentelium nigricans), Slimy Sculpin (Cottus cognatus), Pumpkinseed (Lepomis gibossus), Largemouth Bass (Micropterus salmoides), White Perch (Morone americana), and Fantail Darter (Etheostoma flabellare).
Comments:
Haldeman (1844), using the taxa Alasmodon undulatus, described Alasmidonta undulata, “… dark brown, rayed, a very robust tooth in each valve. 1 ½ inches.”
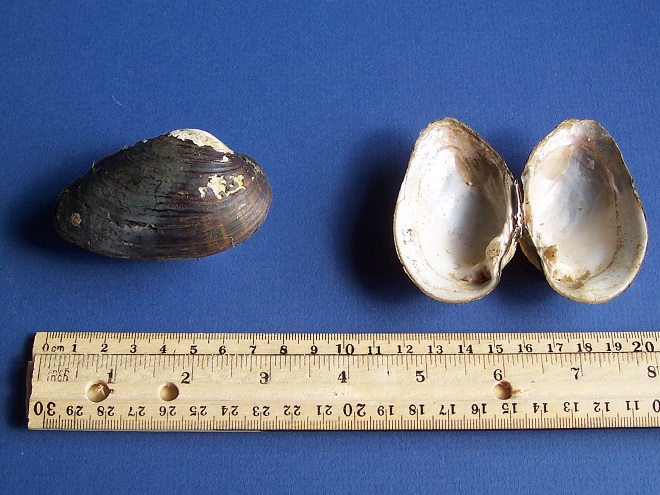
Alasmidonta heterodon
Common Name(s): Dwarf Wedgemussel
Status: Federally Endangered, MD Endangered, PA Endangered
Rank: Pennsylvania S1 (Critically Imperiled), Maryland S1 (Critically Imperiled), Globally G1 (Critically Imperiled) and G2 (Imperiled)
Host Fish known to occur in the Susquehanna and/or its tributaries: Atlantic Salmon (Salmo salar), Brown Trout (Salmo trutta), Banded Killifish (Fundulus diaphanus), Mottled Sculpin (Cottus bairdi), Striped Bass (Morone saxatalis), Tessellated Darter (Etheostoma olmstedi), and Shield Darter (Percina peltata).
Comments: Known in the lower Susquehanna from specimen(s) collected by L. H. Streng on the main stem of the river at Chickies Rock in 1919.



Anodonta implicata
Common Name(s): Alewife Floater
Status:
Rank: Maryland S3 (Vulnerable), Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Alewife (Alosa pseudoharengus), Blueback Herring (Alosa aestivalis), White Sucker (Catastomus commersoni), Pumpkinseed (Lepomis gibbosus), and White Perch (Morone americana).
Comments: A tidal freshwater species found in the Susquehanna watershed only in the area of the mouth of the river in Maryland.


Anodontoides ferussacianus
Common Name(s): Cylindrical Papershell
Status:
Rank: Pennsylvania S2 (Imperiled) and S3 (Vulnerable), Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Sea Lamprey (Petromyzon marinus), Bluntnose Minnow (Pimephales notatus), Common Shiner (Luxilus cornutus), Spotfin Shiner (Cyrpinella spiloptera), White Sucker (Catostomus commersoni), Mottled Sculpin (Cottus bairdii), Bluegill (Lepomis macrochirus), Black Crappie (Pomoxis nigromaculatus), and Largemouth Bass (Micropterus salmoides).
Comments: This species has been recorded in the lower Susquehanna drainage in Lancaster County, Pennsylvania, probably occurring as a native transplant.


Elliptio complanata
Common Name(s): Eastern Elliptio, Flattened Filter Clam
Status:
Rank: Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: American Eel (Anguilla rostrata), Brook Trout (Salvelinus fontinalis), Lake Trout (Salvelinus namaycush), Mottled Sculpin (Cottus bairdii), Slimy Sculpin (Cottus cognatus), Alewife (Alosa pseudoharengus), Banded Killifish (Fundulus diaphanous), Green Sunfish (Lepomis cyanellus), Largemouth Bass (Micropterus salmoides), Pumpkinseed (Lepomis gibbosus), Redbreast Sunfish (Lepomis auritus), Smallmouth Bass (Micropterus dolomieu), White Crappie (Pomoxis annularis), White Perch (Morone americana), and Yellow Perch (Perca flavascens).
Comments: The most common and widespread unionid in the Lower Susquehanna River Watershed.
A laboratory study (Lellis, et. al., 2013) to determine the host fishes for Elliptio complanata from streams on the Atlantic Slope found glochidia from Chesapeake Bay drainages metamorphosed into juvenile mussels on five species of host fish: American Eel (Anguilla rostrata), Brook Trout (Salvelinus fontinalis), Lake Trout (Salvelinus namaycush), Mottled Sculpin (Cottus bairdii), and Slimy Sculpin (Cottus cognatus). American Eel yielded the best results with 13.2 juveniles per fish and a success rate of ≥ 0.90 percent. Subsequently, an effort was begun on the lower Susquehanna to capture migrating catadromous elvers at Conowingo Dam in Maryland and transport them to suitable waterways upstream to support existing populations of Eastern Elliptios and possibly expand their current range back into some of the streams they once occupied.

Haldeman (1844), using the taxa Unio complanatus, described Elliptio complanata, “… compressed, dull brown, inside frequently purple. Young sometimes rayed, extremely variable form, our most common species. 3 inches.”
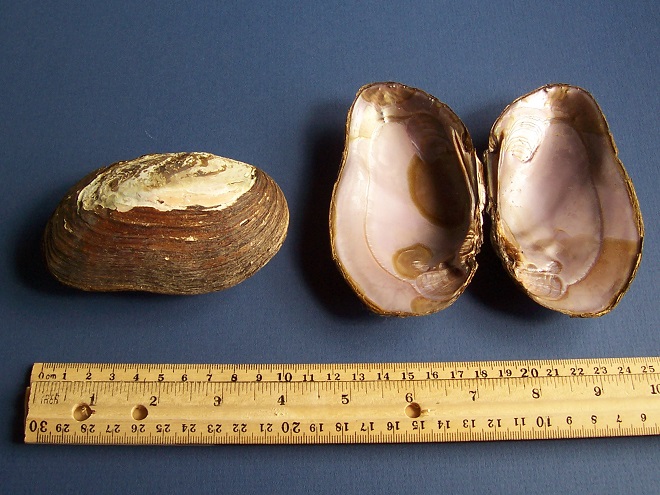
Lampsilis cariosa
Common Name(s): Yellow Lampmussel, Caried Lampmussel
Status:
Rank: Globally G3 (Vulnerable) and G4 (Apparently Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Bluntnose Minnow (Pimephales notatus), White Sucker (Catostomus commersoni), Banded Killifish (Fundulus diaphanous), Chain Pickerel (Esox niger), Pumpkinseed (Lepomis gibbosus), Rock Bass (Amblopites rupestris), Bluegill (Lepomis macrochirus), Black Crappie (Pomoxis nigromaculatus), Largemouth Bass (Micropterus salmoides), Smallmouth Bass (Micropterus dolomieu), White Bass (Morone americana), White Perch (Morone americana), and Yellow Perch (Perca flavescens).
Comments:
Haldeman (1844), using the taxa Unio cariosus, described Lampsilis cariosa. “… shell straw yellow, 3 or 4 inches.”


Lampsilis radiata
Common Name(s): Eastern Lampmussel
Status:
Rank: Pennsylvania S1 (Critically Imperiled), Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Banded Killifish (Fundulus diaphanous), Black Crappie (Pomoxis nigromaculatus), Largemouth Bass (Micropterus salmoides), Rock Bass (Amblopites rupestris), Pumpkinseed (Lepomis gibbosus), Smallmouth Bass (Micropterus dolomieu), White Perch (Morone americana), and Yellow Perch (Perca flavescens).
Comments: The range of the Eastern Lampmussel in the Susquehanna was apparently seriously fragmented, and its numbers precipitously depleted, by various impairment factors including dam construction, siltation, and waste-coal dredging. This species may have been particularly susceptible to the latter due to its large size as a breeding adult.
Haldeman (1844), using the taxa Unio radiata, described Lampsilis radiata, “… covered with broad green bands, 4 or 5 inches.”



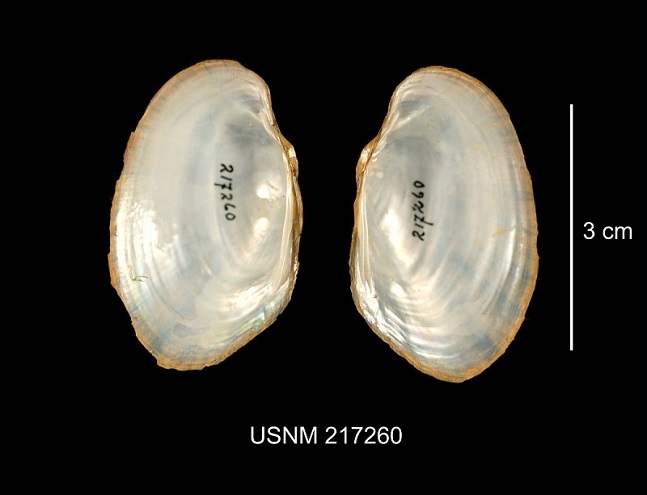
Lasmigona subviridis
Common Name(s): Green Floater
Status: MD Endangered
Rank: Pennsylvania S2 (Imperiled) and S3 (Vulnerable), Maryland S1 (Critically Imperiled), Globally G3 (Vulnerable)
Host Fish known to occur in the Susquehanna and/or its tributaries: None needed? In a lab setting, females have been observed expelling metamorphosed young.
Comments:
Haldeman (1844), using the taxa Unio viridis, described Lasmigona subviridis, “… a small, fragile, brown or green rayed species, with cardinal teeth, compressed and very variable. usually 1 ½ inches.”

Leptodea ochracea
Common Name(s): Tidewater Mucket
Status:
Rank: Maryland S1 (Critically Imperiled) and S2 (Imperiled), Globally G3 (Vulnerable) and G4 (Apparently Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Banded Killifish (Fundulus diaphanous), and White Perch (Morone americana).
Comments: A tidal freshwater species found in the Susquehanna watershed only in the area of the mouth of the river in Maryland.

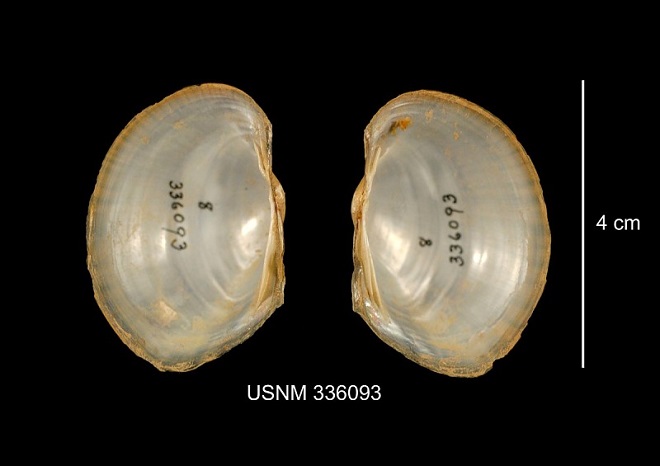
Pyganodon cataracta
Common Name(s): Eastern Floater, Fragile Freshwater Mussel
Status:
Rank: Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Common Carp (Cyprinus carpio), White Sucker (Catostomus commersoni), Pumpkinseed (Lepomis gibbosus), and Rock Bass (Ambloplites rupestris).
Comments: Buoyant and more tolerant of silt than other river mussels, it is the most likely species to be found surviving in spring-fed ponds, farmland streams, and millponds choked with legacy sediments.
Haldeman (1844), using the taxa Anodon cataractus, described Pyganodon cataracta, “… bright green, rayed: delicate. 4 or 5 inches.”



Strophitus undulatus
Common Name(s): Creeper
Status:
Rank: Maryland S2 (Imperiled), Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Spotfin Shiner (Cyrpinella spiloptera), Bluntnose Minnow (Pimephales notatus), Longnose Dace (Rhinichthys cataractae), Central Stoneroller (Campostoma anomalum), Creek Chub (Semotilus atromaculataus), Yellow Bullhead (Ameriurus natalis), Largemouth Bass (Micropterus salmoides), Rock Bass (Ambloplites rupestris), Bluegill (Lepomis macrochirus), White Crappie (Pomoxis annularis), Walleye (Stizostedion vitreum), Banded Darter (Etheostoma zonale), and Fantail Darter (Etheostoma flabellare).
Comments:
Haldeman (1844), using the taxa Anodon undulatus, described Strophitus undulatus, “… dark brown, hinges slightly thickened having a tendency to form a slight pair of teeth. 2 or 3 inches.”

Villosa iris
Common Name(s): Rainbow Mussel
Status:
Rank: Globally G5 (Secure)
Host Fish known to occur in the Susquehanna and/or its tributaries: Rock Bass (Ambloplites rupestris), Smallmouth Bass (Micropterus dolomieu), and Largemouth Bass (Micropterus salmoides).
Comments: A native transplant to the Susquehanna.


OTHER FRESHWATER BIVALVE MOLLUSKS
Order-Sphaeriida
Family Sphaeriidae
Sphaeriidae is a family of small freshwater bivalves never exceeding one inch in length. Three genera are found locally—Pisidium, the Peashell or Pill Clam; Sphaerium, the Short-siphoned Fingernail Clam; and Musculium, the Long-siphoned Fingernail Clam. Sphaeriidae are most often found in sand and gravel in flowing waterways. Sphaeriidae, particularly Pisidium, are known to climb vegetation and may prefer habitats with emergent and submerged aquatic plants. A few species will inhabit ponds and vernal pools. In the Susquehanna River and its tributaries, populations of Sphaeriidae may have been reduced by invasive populations of the non-native Asiatic Clam (Corbicula fluminea). Stream segments suffering from heavy silt deposits rarely support significant populations of Sphaeriidae clams.


Order-Venerida
Family Corbiculidae
The Asiatic Clam, Corbicula fluminea, native to East Asia, is a member of the family Corbiculidae. Asiatic Clams produce veliger larvae that distribute throughout the water column. Unlike the Unionidae, Corbiculidae larvae require no host fish to ensure survival. Asiatic Clams are able to distribute quickly into new areas of suitable habitat.
In North America, Corbicula fluminea was introduced to the Columbia River in Washington by Chinese immigrants in 1938. During the 1960s, they arrived in the Chesapeake Bay watershed, reaching the Potomac River by 1975 (Lippson and Lippson 1997). They invaded the Susquehanna River in Lancaster County in the mid-1980s. By 1990, Corbicula fluminea was the most conspicuous macroinvertebrate in the river. Today, shells of expired clams blanket the bottom of the Susquehanna from shore to shore in many areas. Downstream of Conewago Falls, searching a cubic foot of submerged sand will often yield 30 to 50 individual live Asiatic Clams. The impact of their enormous biomass on other benthic organisms could be studied at length. Electric generating facilities on the lower Susquehanna River have seen dense colonies of Corbicula fluminea foul their cooling systems. Resulting shutdowns and repairs have significant economic impact. Asiatic Clams have invaded many of the Susquehanna’s tributaries. Fortunately, they are not as invasive in cooler, fast-moving, high-gradient headwaters.


Order-Myida
Family Dreissenidae
Members of the family Dreissenidae—the Zebra Mussel, Dreissena polymorpha, and the Quagga Mussel, Dreissena rostriformis bugensis—are invasive freshwater mollusks of particular concern in the Lower Susquehanna River Watershed. Both species are often known collectively as “zebra mussel”. Using the name “clam” instead of “mussel” to describe a member of the bivalve family Unionidae would seem practical to most observers. In comparison, Dreissena are “mussels” in the familiar sense, having byssal threads to firmly attach to solid surfaces and to each other, much like the familiar mussels found on pilings and rocks on the Atlantic coastline. Creating massive, clumped colonies up to three feet in depth, Dreissena mussels cause millions of dollars in damage at power generating stations, manufactories, and other facilities with freshwater intakes. Removal of Dreissena bivalves leads to operational shutdowns, high maintenance costs, and often the need for chemical treatment. Some facilities faced with burdening populations of zebra mussels must be reengineered and reconstructed to compensate for their presence (O’Neill 1996).


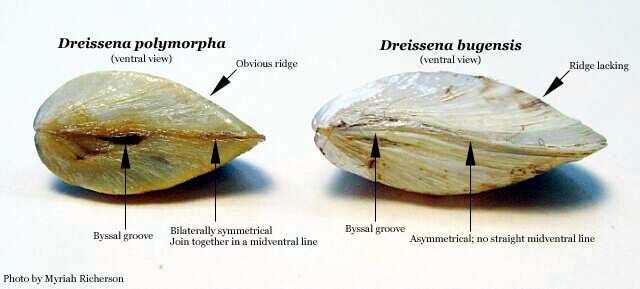
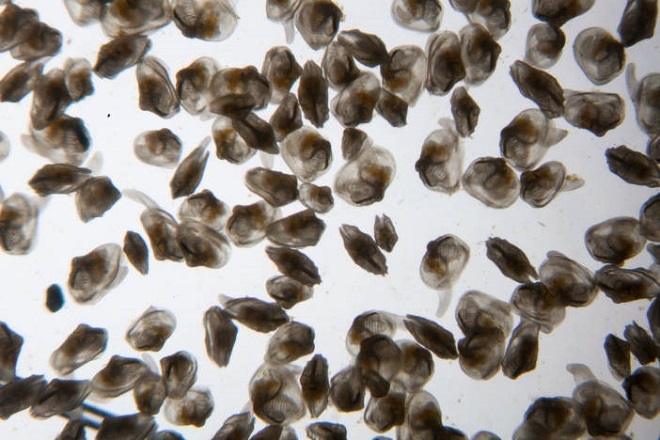
Dreissena mussel larvae (veligers) are believed to have arrived in North America as stowaways on ships. Freshwater containing veligers was pumped aboard these vessels as ballast prior to leaving an infected Eurasian port, then discharged upon arrival in a Great Lakes port. Adult mussels may have arrived on anchor chains and other equipment not exposed to salt water during the voyage (O’Neill 1996).
Zebra mussels are native to the Caspian and Black Seas. Their dispersal as a non-native invasive species was rapid. Shaw et al. (2004) tracks the arrival of Dreissena from infected ports in Eurasia. They were first noted in North America at Lake St. Clair on the Michigan/Ontario border in 1988. By 1989, Dreissena were well established in sizeable colonies on the Great Lakes. By 1991, they had extended their range into many of the major waterways of the northeastern United States and eastern Canada.
The first occurrence of Dreissena in the Susquehanna River watershed was of veligers at a sampling point at Goudy Station in Endicott, New York, in 1992. No adults were found. In Madison County, New York, a zebra mussel colony was found in the outflow of Eaton Brook reservoir upstream of the Upper Chenango River in 2000. In 2002, a reproducing population was found in Canadarago Lake in Ostego County, New York. Canadarago Lake is frequented by recreational boats which may have acted as vectors to infect the lake with Dreissena. In 2004, Armstrong (2004) found adult and juvenile Zebra Mussels, Dreissena polymorpha, in Goodyear Lake, an impoundment on the Susquehanna River main stem near Cooperstown, New York—downstream of Canadarago Lake. It is believed Goodyear Lake was colonized by veligers from Canadarago Lake.

Promptly detecting and controlling isolated populations of zebra mussels can be a practice of significant economic advantage. In the Chesapeake Bay watershed, the Millbrook Quarry in Prince William County, Virginia, is believed to have been infected with Dreissena by SCUBA divers or other recreational users. After several years of establishment, the mussels were eradicated in late 2006 using 174,000 gallons of Potassium Chloride solution at a cost of $365,000 (see Millbrook Quarry Zebra Mussel Eradication 2006). The elimination of Dreissena at the Millbrook Quarry will prevent their spread to neighboring waterways and will save operators of local water supply and power generating facilities millions of dollars in treatment, repair, and shutdown costs.
In 2000, zebra mussels (Dreissena species) were found at the Willow Springs Diving Park in the Richland Quarry near Myerstown, Lebanon County, Pennsylvania (Shaw et al. 2004). This SCUBA facility is located adjacent to the Lower Susquehanna River Watershed in the Schuylkill River drainage basin.
During 2008 and 2009, Quagga Mussels (Dreissena rostriformis bugensis) were found in Billmeyer Quarry, a flooded dolomite operation near Bainbridge, Lancaster County, Pennsylvania. The quarry, which happens to be located along the Susquehanna next door to Professor Samuel Steman Haldeman’s birthplace, was in use at the time as a SCUBA diving facility. Following their discovery, the mussels were eradicated.

In 2008, during a drawdown of the Muddy Run Pumped Storage reservoir along the eastern shore of the Susquehanna in southern Lancaster County, Pennsylvania, the valves of dead Zebra Mussels (Dreissena polymorpha) were found. This impoundment, which is frequented by recreational boaters, apparently functioned as a source for establishment of the species in the lower Susquehanna. During the following year, veligers were discovered downriver nearby along the western shore within the intake canals of Units 2 and 3 at the Peach Bottom Atomic Power Station. In 2010, an adult was found there and subsequently a population became established throughout the adjacent segment of the Susquehanna River known as Conowingo Pond. The range of that population now extends to the mouth of the Susquehanna on Chesapeake Bay at Havre de Grace, Maryland.

THE VALUE OF MONITORING AND PROTECTING NATIVE MUSSELS
Monitoring Unionidae mussel communities provides data that is useful in efforts to protect them, their aquatic habitats, and the quality of the water they inhabit. Examination of unionid populations may be useful when assessing the impact of stream impairment and land use on benthic organisms. Because some species of freshwater mussels can live for decades and, during that time, bioaccumulate a number of pollutants, they can function as long-term indicators of stream health. On waterways where sediment and nutrient reduction projects are being implemented, the population densities and species diversity of native freshwater mussel communities may provide insight on the effectiveness of conservation work. Pollution mitigation techniques found beneficial to the unionids could be utilized in other areas where mussels and other benthic fauna are imperiled. And of course, protecting and restoring populations of Unionidae mussels promotes purification of the streams, rivers, and lakes they inhabit. They are, after all, nature’s own water filters.
CONSERVATION PRACTICES BENEFICIAL TO NATIVE BIVALVES
MONITORING


CONSERVATION FARMING




DAM AND LEGACY SEDIMENT REMOVAL








FISH PASSAGE


RIPARIAN BUFFERS



AQUATIC VEGETATION



WETLANDS


I’M WORRIED ABOUT THE BEAVER


STORMWATER MANAGEMENT






…AND FINALLY, ONE LAST PIECE OF ADVICE

SOURCES
Armstrong, Samantha. 2004. Survey of veliger and adult zebra mussels (Dreissena polymorpha) in Goodyear Lake. SUNY Oneonta Biological Field Station, SUNY Oneonta, NY. 7pp.
Bogan, Arthur, and Ashton, Mathew. 2016. Manual of the Freshwater Bivalves of Maryland. Maryland Department of Natural Resources. Annapolis, MD. 63 pp.
Bruckhart, H. G. 1869. Conchology of Lancaster County. J. I. Mombert. An Authentic History of Lancaster County in the State of Pennsylvania. J. E. Barr and Company. Lancaster, PA. pp. 517-518.
Clarke, Arthur H., Jr., and Berg, Clifford O. 1959. The Freshwater Mussels of Central New York; With an Illustrated Key to the Species of Northeastern North America. Cornell University Agricultural Experiment Station. Memoir 367. 79 pp.
Haldeman, Samuel S. 1841. Commentary on Unio viridis and an Experiment in Transplanting Unio from the Ohio to the Susquehanna. Proceedings of the Academy of Natural Sciences. 1: 104.
Haldeman, Samuel S. 1841. Commentary on a Specimen of Unio crassus from the Susquehanna. Proceedings of the Academy of Natural Sciences. 3:14-16.
Haldeman, Samuel S. 1844. Enumeration of the recent freshwater Mollusca which are common to North America and Europe; with observations on species and their distribution. Boston Journal of Natural History. 4:468-484.
Haldeman, Samuel S. 1844. Sketch of the natural history of Lancaster County, Penna. Rupp’s History of Lancaster County, I. D. Rupp. Chapter XIII.
Haldeman, Samuel S. 1845. A Monograph of the Freshwater Univalve Mollusca of the United States. E. G. Dorsey, Philadelphia, PA. 2 volumes. 231 pp.
Lellis, William A.; St. John White, Barbara; Cole, Jeffrey C.; Johnson, Connie S.; Devers, Julie L.; van Snik Gray, Ellen; and Galbraith, Heather S. 2013. Newly Documented Host Fishes for the Eastern Elliptio Mussel Elliptio complnata. Journal of Fish and Wildlife Management. 4(1):75-85.
Lippson, Alice Jane, and Lippson, Robert L. 1997. Life in Chesapeake Bay. Second Edition. The Johns Hopkins University Press, Baltimore, MD. 294 pp.
Millbrook Quarry Zebra Mussel Eradication. Virginia Department of Game & Inland Fisheries. 2006. http://www.dgif.state.va.us/zebramussels/
Moser, G. Andrew. 1993. Dwarf Wedge Mussel (Alasmidonta heterodon) Recovery Plan. Annapolis Field Office, U.S. Fish and Wildlife Service Northeast Region, Annapolis, MD.
Nightingale, Betsy; Walsh, Mary; Homans, D. David; Evans, Ryan; Bond, Emily; and Deeds, Jeremy. 2004. Pennsylvania Aquatic Community Classification Project. The Pennsylvania Natural Heritage Program, Final Phase I Report. Chapter 4: Mussel Community Classification. pp. 152-168.
O’Neill, Charles R., Jr. 1996. The Zebra Mussel Impacts and Control. Cornell Cooperative Extension Information Bulletin 238. 62 pp.
Pennsylvania Science Office, The Nature Conservancy. 2005. A Natural Areas Inventory of Dauphin County, Pennsylvania. Update-2005. The Nature Conservancy, Middletown, PA. 142 pp.
Shaw, Tony, et al. 2004. Zebra Mussels (Dreissena polymorpha) in Chesapeake Bay Watershed: A Regional Management Plan. Final Draft. The Regional Dreissena polymorpha Working Group. 38 pp.
Stambaugh, John W., Jr., and Denoncourt, Robert F. 1974. A preliminary Report on the Conewago Creek Faunal Survey, Lancaster County, Pennsylvania. Proceedings of the Academy of Science. 48:55-60.
Stranahan, Susan Q. 1993. Susquehanna, River of Dreams. The Johns Hopkins University Press. Baltimore, MD. 322 pp.
Strayer, D. L., and Jirka, K. J. 1997. The Pearly Mussels of New York State. The New York State Education Department, Albany, NY. 113 pp.
Van Diver, Bradford B. 1990. Roadside Geology of Pennsylvania. Mountain Press Publishing Company. Missoula, MT. 352 pp.

